Cbr4 Electron Dot Structure
Learn to determine if CBr4 is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structure and then us.

Cbr4 Electron Dot Structure
May 24, 2023 by Jay Rana CBr4 is a NONPOLAR molecule. But why? And how can you say that CBr4 is a nonpolar molecule? Want to know the reason? Let's dive into it! CBr4 is a NONPOLAR molecule because all the four bonds (C-Br bonds) are identical and CBr4 has symmetrical geometry which cancels out the bond polarity.
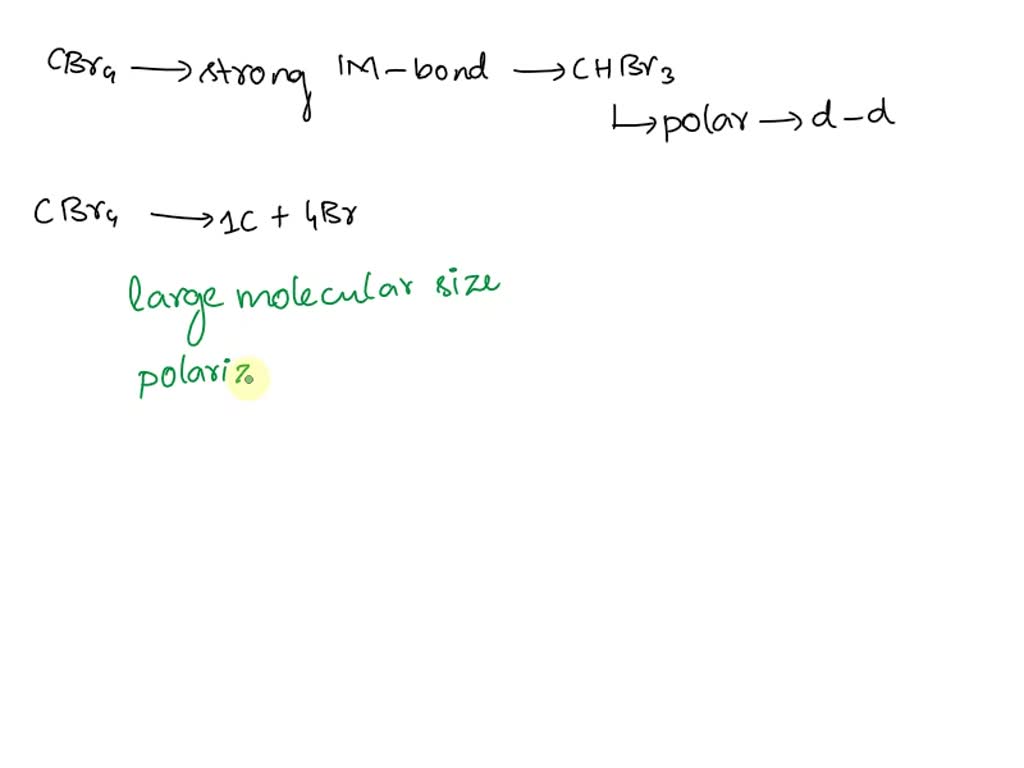
SOLVED CBr4 has stronger intermolecular bonds than CHBr3, indicating
When you place a molecule with an electric dipole in an electric field, a force acts to turn the molecule so that the positive and negative ends line up with the field. The magnitude of the turning force is given by the formula. µ = q × d. where q is the amount of charge and d is the distance between the two charges. µ is the turning moment.
Cbr4 Electron Dot Structure
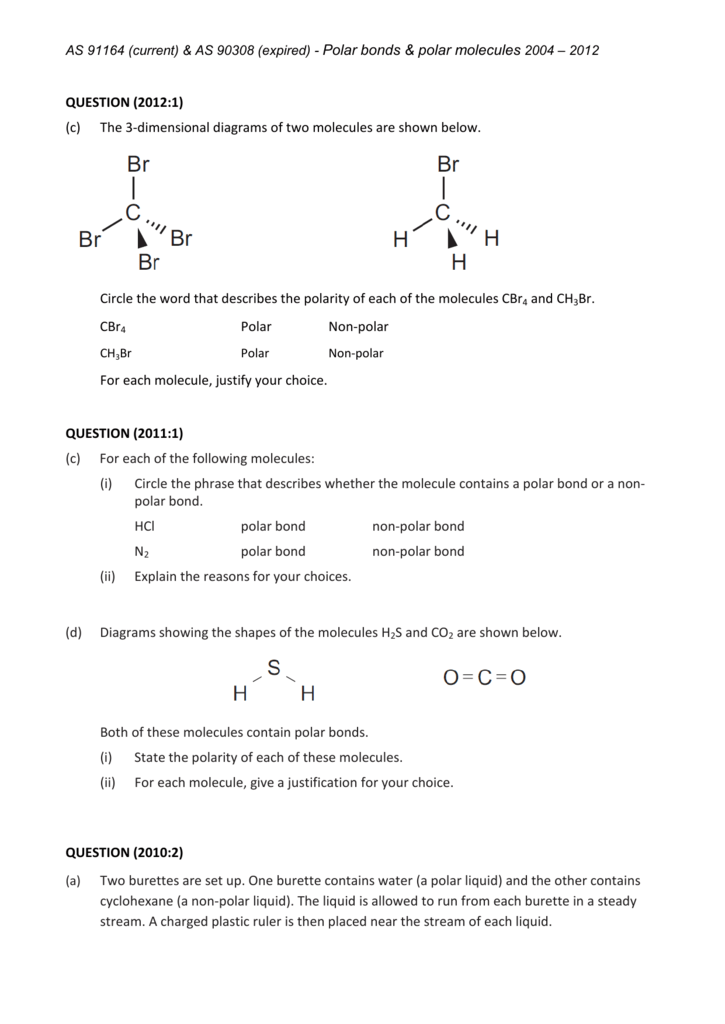
The CBr4 molecule is non-polar.. Both CBr4 and CH3Br have four regions of electrons around the central carbon atom. These are all bonding electron regions (clouds) so the shape of both molecules is tetrahedral. The C-Br bond is polar due to the difference in electronegativity between C and Br. Is carbon polar and nonpolar?
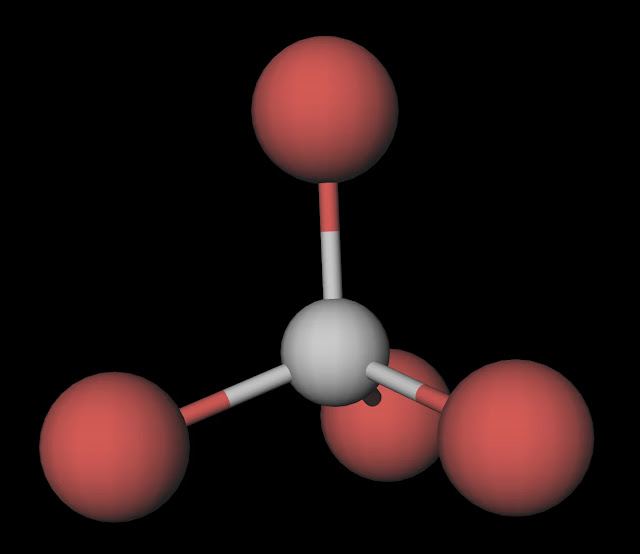
MakeTheBrainHappy Is CBr4 Polar or Nonpolar?
The C-O bond is considerably polar. Although C and S have very similar electronegativity values, S is slightly more electronegative than C, and so the C-S bond is just slightly polar. Because oxygen is more electronegative than sulfur, the oxygen end of the molecule is the negative end. Chloromethane, CH 3 Cl, is another example of a polar.
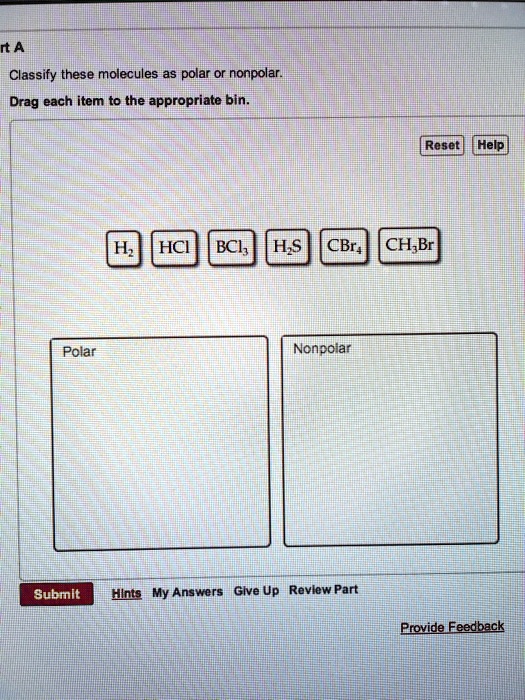
SOLVED rtANClassify these molecules as polar or nonpolar Drag each
Why is a C-Br bond considered polar? From electronegativity considerations, both carbon and bromine have very similar electronegativities - 2.5 and 2.8 - respectively. Nonetheless, I am told that such a bond would be polar. We generally classify bond between atoms with EN differences < 0.5 as non-polar; why would C-Br be an exception? bond polarity

Is CBr4 Polar or Nonpolar? Techiescientist
CBr4 (Carbon tetrabromide) is nonpolar in nature because of the symmetrical arrangement of four bromine atoms around carbon. As a result, the dipoles of the C-Br bond get canceled by each other resulting in CBr4 a nonpolar molecule.

Ch4 Polar Or Nonpolar / Solution Is The Ch4 A Polar Or Non Polar Chemistry
The CBr4 molecule is non-polar due to its symmetrical tetrahedral geometry. The four bromine atoms are arranged symmetrically around the central carbon atom, with each bond angle at 109.5 degrees. This results in an even distribution of charge throughout the molecule, with no net dipole moment.

Nama Senyawa Cbr4 Brain
IUPAC Standard InChIKey:HJUGFYREWKUQJT-UHFFFAOYSA-N. CAS Registry Number: Chemical structure: This structure is also available as a 3d SD file The 3d structure may be viewed using. Other names: Methane, tetrabromo-; Carbon bromide (CBr4); Methane tetrabromide; Tetrabromomethane; CBr4; Carbon bromide; Bromid uhlicity; UN 2516; NSC 6179.

CBR4 Is Best Or Not? SubscribeMe YouTube
The Lewis structure of CBr4 molecule contains 24 non-bonding electrons that is 12 lone pairs (3 lone pairs are present on each Bromine atom). Details of CBr4 CBr4 lewis structure How to draw Lewis dot structure for CBr4? Following are the steps to follow to draw the Lewis structure of CBr4 molecule
Ch4 Polar Or Nonpolar Methane Ch4 Polar Or Nonpolar / Is Ch4 Polar Or
May 27, 2023 by Jay Rana CBr4 is a covalent (nonpolar covalent) compound because when one nonmetal combines with another nonmetal, it usually forms a covalent compound. Here, C is a nonmetal and Br is also a nonmetal. So when they combine, it forms a covalent compound.

Is CBr4 Polar or Nonpolar? Techiescientist
The molecule CBr4 is non-polar. This is because the charges from the bromine atoms cancel out, resulting a neutral charge. The molecule CBr is called tetrabromomethane, but is commonly known as carbon bromine.
Solved 13) Choose the most polar molecule a) CH3Br b) CBr4
Carbon tetrabromide (CBr4) is a non-polar molecule. The central carbon (C) atom in the CBr4 molecule is surrounded by four bromine (Br) atoms via single covalent bonds, forming a symmetric tetrahedral molecule. The electronegativity of the bromine (Br) atom is slightly more than the carbon (C) atom.

Is CBr4 Polar or Nonpolar? (Carbon Tetrabromide) YouTube
Carbon tetrabromide Carbon tetrabromide, CBr 4, also known as tetrabromomethane, is a bromide of carbon. Both names are acceptable under IUPAC nomenclature . Production CBr 4 can be obtained by the bromination of methane. The byproducts include other brominated methanes ( methyl bromide, dibromomethane and bromoform) and hydrogen bromide.
CH Br Polar Nonpolar
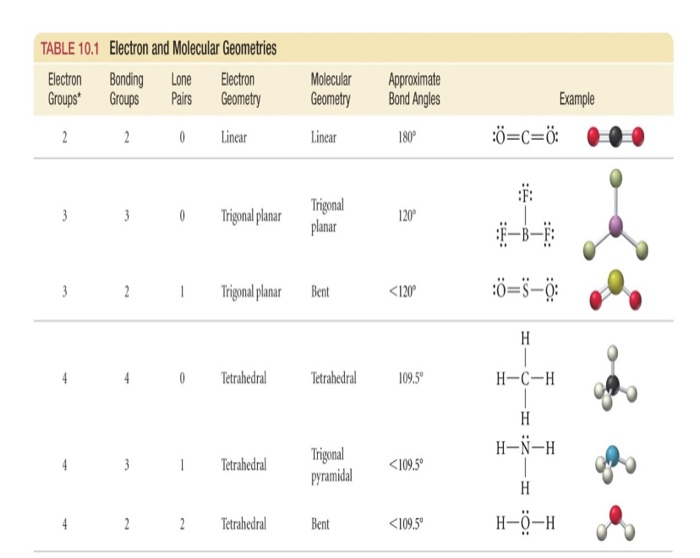
The VSEPR Model VSEPR Theory Practice Problems Hybridization of Atomic Orbitals sp , sp2 , sp3 , sp3d, and sp3d2 Hybridization Practice Problems CBr4 Polar or Nonpolar? Even though the C-Br bond is polar, the symmetrical shape of the molecule makes it nonpolar as it has no net dipole.
Cbr4 Lewis Structure
Other names: Methane, tetrabromo-; Carbon bromide (CBr4); Methane tetrabromide; Tetrabromomethane; CBr4; Carbon bromide; Bromid uhlicity; UN 2516; NSC 6179 Permanent link for this species. Use this link for bookmarking this species for future reference. Information on this page: Notes; Other data available: Gas phase thermochemistry data